Researchers face a fundamental challenge as they seek to scale up human tissue regeneration from small lab samples to full-size tissues, bones, even whole organs to implant in people to treat disease or traumatic injuries: how to establish a vascular system that delivers blood deep into the developing tissue.
Current bioengineering techniques, including 3-D printing, can’t fabricate the branching network of blood vessels down to the capillary scale that are required to deliver the oxygen, nutrients, and essential molecules required for proper tissue growth. To solve this problem, a multidisciplinary research team at Worcester Polytechnic Institute (WPI), the University of Wisconsin-Madison, and Arkansas State University-Jonesboro have successfully turned to plants. They report their initial findings in the paper “Crossing kingdoms: Using decellularized plants as perfusable tissue engineering scaffolds” published online in advance of the May 2017 issue of the journal Biomaterials.
Plants and animals exploit fundamentally different approaches to transporting fluids, chemicals, and macromolecules, yet there are surprising similarities in their vascular network structures,” the authors wrote. “The development of decellularized plants for scaffolding opens up the potential for a new branch of science that investigates the mimicry between plant and animal.”
In a series of experiments, the team cultured beating human heart cells on spinach leaves that were stripped of plant cells. They flowed fluids and microbeads similar in size to human blood cells through the spinach vasculature, and they seeded the spinach veins with human cells that line blood vessels. These proof-of-concept studies open the door to using multiple spinach leaves to grow layers of healthy heart muscle to treat heart attack patients.
Other decellularized plants could provide the framework for a wide range of tissue engineering technologies. “We have a lot more work to do, but so far this is very promising,” said Glenn Gaudette, PhD, professor of biomedical engineering at WPI and corresponding author of the paper. “Adapting abundant plants that farmers have been cultivating for thousands of years for use in tissue engineering could solve a host of problems limiting the field.”
In addition to Gaudette, the WPI research team includes Tanja Dominko, PhD, DVM, associate professor of biology and biotechnology, who studies molecular mechanisms of human cell development; Pamela Weathers, PhD, professor of biology and biotechnology, a plant biologist; and Marsha Rolle, PhD, associate professor of biomedical engineering, who focuses on vasculature tissue engineering. The collaborative team also includes human stem cell and plant biology researchers at Wisconsin and Arkansas. “This project speaks to the importance of interdisciplinary research,” Gaudette said. “When you have people with different expertise coming at a problem from different perspectives, novel solutions can emerge.”
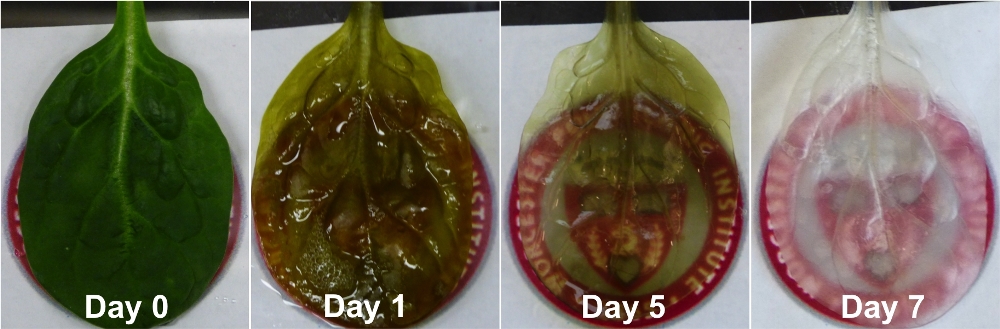
The paper’s first author is Joshua Gershlak, a graduate student in Gaudette’s lab, who helped design and conduct the experiments, and who developed an effective process for removing plant cells from spinach leaves by flowing or “perfusing” a detergent solution through the leaves’ veins. “I had done decellularization work on human hearts before,” Gershlak said, “and when I looked at the spinach leaf its stem reminded me of an aorta. So I thought, let’s perfuse right through the stem. We weren’t sure it would work, but it turned out to be pretty easy and replicable. It’s working in many other plants.”
When the plant cells are washed away what remains is a framework made primarily of cellulose, a natural substance that is not harmful to people. “Cellulose is biocompatible (and) has been used in a wide variety of regenerative medicine applications, such as cartilage tissue engineering, bone tissue engineering, and wound healing,” the authors wrote.
In addition to spinach leaves, the team successfully removed cells from parsley, Artemesia annua (sweet wormwood), and peanut hairy roots. They expect the technique will work with many plant species that could be adapted for specialized tissue regeneration studies. “The spinach leaf might be better suited for a highly vascularized tissue, like cardiac tissue, whereas the cylindrical hollow structure of the stem of Impatiens capensis (jewelweed) might better suit an arterial graft. Conversely, the vascular columns of wood might be useful in bone engineering due to their relative strength and geometries,” the authors wrote.
Using plants as the basis for tissue engineering also has economic and environmental benefits. “By exploiting the benign chemistry of plant tissue scaffolds,” they wrote, “we could address the many limitations and high costs of synthetic, complex composite materials. Plants can be easily grown using good agricultural practices and under controlled environments. By combining environmentally friendly plant tissue with perfusion-based decellularization, we have shown that there can be a sustainable solution for pre-vascularized tissue engineering scaffolds.”
At WPI, the research continues along several lines, Gaudette said, with studies to optimize the decellularization process and further characterize how various human cell types grow while they are attached to, and are potentially nourished by, plant-based scaffolds. Also, engineering a secondary vascular network for the outflow of blood and fluids from human tissue will be explored. On April 7, 2017, Gershlak will present the technology and early results as an invited speaker at the National Academy of Inventors inaugural Student Innovation Showcase in Boston, where he will detail the work for more than 200 accomplished inventors and technology commercialization leaders.








